
Department of Chemistry |
1220 Jesse D. Jones Hall |
|---|
|
Fannin LaboratoryResearch projects in our laboratory are divided into 4 major areas: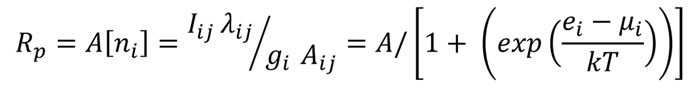
1. Plasma Modeling: This is the longest ongoing research area in the laboratory dating from the late 1980's. It is an outgrowth of research at U.C. in which a mono-thermal model was developed for the excited electronic states of a helium in a steady state reduced pressure ICP system[1]. At MSU this model was expanded to include other reduced pressure plasma systems[2] , electrons in the plasma[3] , and determination of temperature in reduced and atmospheric pressure plasmas [4,5] . This model was quantitatively compared to existing models for the determination of excitation temperatures [6] and refinements to fitting parameters of the model were made [7]; additionally, the model was utilized to predict variations in detection limits in plasma systems[8] .
Interest in this area had waned somewhat, until the use of this model once in 2003 and again in four publications in 2007 by independent researchers on completely different plasma systems. We are currently attempting to refine the calculation of the chemical potential and explore the use of this model for a variety of plasma systems.
2. Instrument Development: Instrument development efforts have focused on the use of reduced pressure ICPs as both optical sources and ion sources. Attempts have been made to utilize these sources in a variety of techniques including FIA, and hyphenated with TGAs, and GCs with varying degrees of success. Only a few of these experiments were successful enough to warrant publication [1-4]
3. Chemical Consulting and Analytical Support: As a professional courtesy and as part of the university's economic development mandate, our laboratory has provided a wide range of services including product development, failure analysis, and chemical and physical characterization of materials. We have provided services for industrial companies, including RT Vanderbilt, Alcan Composites, ISP, Lockheed Martin and Degussa, as well as, for governmental agencies, such as, the Kentucky Geological Survey, Tennessee Wildlife Resources Agency, and the United States Navy[]. The results from the last collaboration were featured in USA Today.
4. Small System Thermodynamics: This is the newest area of research and is an outgrowth of an early Journal of Chemical Education paper with Mark Masthay involving positive and negative temperature in two level systems[1]. Currently, our efforts in collaboration with Dr. Masthay's laboratory at the University of Dayton are centered around examination of various expressions for entropy and the corresponding temperature(s) in systems with varying numbers of particles and fields.
|